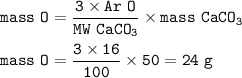The number of oxygen atoms = 3
Mass = 24 g
Further explanation
The formula of a compound shows the composition of the constituent elements
CaCO₃ is composed of 3 types of elements, namely Ca, C and O
The amounts of each of these elements in the compound CaCO₃:
So the number of oxygen atoms = 3
mass of Oxygen :