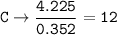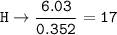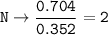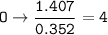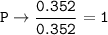The empirical formula : C₁₂H₁₇N₂O₄P
Further explanation
The empirical formula is the smallest comparison of atoms of compound =mole ratio of the components
The principle of determining empirical formula
- Determine the mass ratio of the constituent elements of the compound.
- Determine the mole ratio by by dividing the percentage by the atomic mass
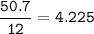
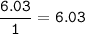
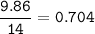
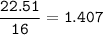
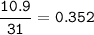
Divide by the smallest mole ratio(0.352)
C : H : N : O : P