Answer:

Step-by-step explanation:
Mass of a proton,
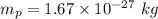
Mass of an electron,
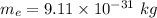
The distance between the electron and the proton is,
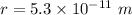
We need to find the mutual attractive gravitational force between the electron and proton. The gravitational force is given by :
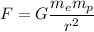
Where G is the universal Gravitational constant
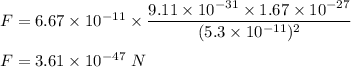
So, the force between the electron and proton is
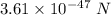 .
.