Answer:

Step-by-step explanation:
The formula of density is:
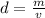
If the formula is rearranged for volume, it becomes:
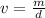
The mass of the platinum is 38.5 grams.
The density is 21.5 grams per cubic centimeter.
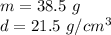
Substitute the values into the formula.
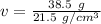
Divide. Note that the grams (g) will cancel each other out.
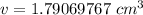
Round to 2 decimal places (hundredth place). The 0 in the thousandth place tells us to leave the 9 in the hundredth place.
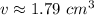
The volume is about 1.79 cubic centimeters.