Answer:
4.5moles
Step-by-step explanation:
Given parameters:
Mass of Na₃P = ?
Unknown:
Number of moles = ?
Solution;
The number of moles in a given substance can be derived from the expression below;
Number of moles =

Molar mass of Na₃P = 3(23) + 31 = 100g/mol
Number of moles =
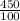 = 4.5moles
= 4.5moles