Initial volume of graduated cylinder = 30.0 mL
Final volume of graduated cylinder after adding rock = 75.0 mL
Volume of the rock = Final volume of graduated cylinder after adding rock - Initial volume of graduated cylinder
= (75.0 - 30.0) mL
= 45.0 mL
Mass of the rock = 55.0 g
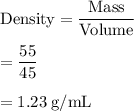
 Density of the rock = 1.23 g/mL
Density of the rock = 1.23 g/mL