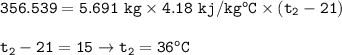The final temperature = 36 °C
Further explanation
The balanced combustion reaction for C₆H₆
2C₆H₆(l)+15O₂(g)⇒ 12CO₂(g)+6H₂O(l) +6542 kJ
MW C₆H₆ : 78.11 g/mol
mol C₆H₆ :
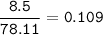
Heat released for 2 mol C₆H₆ =6542 kJ, so for 1 mol
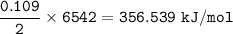
Heat transferred to water :
Q=m.c.ΔT