Answer:
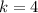
Explanation:
Using the diagram, we can conclude that the given angles are vertical angles. Vertical angles are opposite angles that are formed when two lines intersect. Vertical angles are always congruent.
Using this information we can set up an equation.
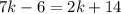
Subtract 2k from both sides.
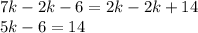
Add 6 to both sides
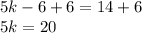
Divide both sides by 5
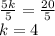
CHECK:
7(4) - 6 = 28 - 6 = 22
2(4) + 14 = 8 + 14 = 22
22 = 22