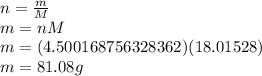Answer:
81.08g of H
 O will be produced.
O will be produced.
Step-by-step explanation:
Write down the balanced chemical equation:
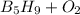 ⇒
⇒
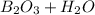
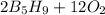 ⇒
⇒
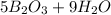
Determine the limiting reagent:
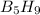 :- 126/63.12646 = 1.995993 mol
:- 126/63.12646 = 1.995993 mol
1.995993/2 = 0.9979965
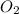 :- 192/31.9988 = 6.000225 mol
:- 192/31.9988 = 6.000225 mol
6.000225/12 = 0.50001875
Therefore,
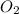 , is the limiting reagent.
, is the limiting reagent.
Use stoichiometry ratios to determine the number of moles of water produced:
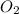 :
:
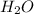
12 : 9
6.000225 : 4,500168756328362
Use the mole formula to calculate the mass of water produced: