Answer:
CH2
Step-by-step explanation:
We are given that a compound is 14.40% hydrogen and 85.60% carbon.
Let the mass of the substance be 100g.
Mass of the hydrogen: 14.40% of 100 = 14.40 g
Mass of the carbon: 85.60% of 100 = 85.60 g
Now, let's find the moles of hydrogen:
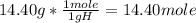
Moles of carbon:
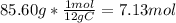
Let's put these in a ratio and simplify:
7.13 mole C: 14.40 mole H
1 mole C: 2 mole H
Therefore, the empirical formula of this compound is CH2.
Hope this helps!! If you have any questions about my work, please let me know in the comments!