Answer:
There are approximately 8.183 × 10²³ units of PbO₂ in 325 grams of PbO₂
Step-by-step explanation:
The given mass of PbO₂ = 325 grams
The molar mass of PbO₂ = 239.2 g/mol
The number of moles of PbO₂ in 325 grams of PbO₂ is given by the formula for the number of moles of a chemical compound in a given sample of the compound as follows;
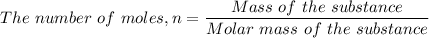
For the given PbO₂ compound, in the question, we have;
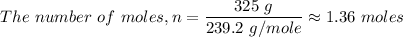
The number of units of PbO₂ in one mole is given by the Avogadro's number as follows;
The number of units of PbO₂ per mole = 6.023 × 10²³ units
The number of units of PbO₂,
 in 1.36 mole of PbO₂ is therefore;
in 1.36 mole of PbO₂ is therefore;
 = 1.36 × 6.023 × 10²³ units ≈ 8.183 × 10²³ units of PbO₂
= 1.36 × 6.023 × 10²³ units ≈ 8.183 × 10²³ units of PbO₂
The number of units of PbO₂, in 1.36 mole of PbO₂ ≈ 8.183 × 10²³ units.