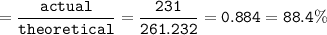The percent yield : 88.4%
Further explanation
Reaction
Salicylic acid (C₇H₆O₃) reacts with acetic anhydride (C₄H₆O₃) to form acetylsalicylic acid (C₉H₈O₄)
2C₇H₆O₃(aq)+C₄H₆O₃(aq)⇒2C₉H₈O₄(aq) + H₂O(l)
MW C₇H₆O₃ : 138.12 g/mol
mol C₇H₆O₃
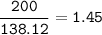
MW C₉H₈O₄ : 180.16 g/mol
mol C₇H₆O₃ = mol C₉H₈O₄ (mol ratio from equation 2:2), so mass of C₉H₈O₄
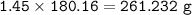
percent yield :