Answer:
80mg
Step-by-step explanation:
Given parameters:
Density of Platinum metal = 21.4g/mL
Dimension of the metal = 3cm by 0.03m by 40mm
Unknown:
Weight of the metal in milligrams?
Solution:
Density is the mass per unit volume of a substance.
Mathematically;
Density =

Volume of the platinum metal = l x w x h
Let us convert the dimension of the metal to the same unit;
3cm x 0.03m x 40mm
convert to cm;
100m = 1cm
0.03m =
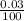 = 0.0003cm
= 0.0003cm
-- 40mm;
1mm = 0.1cm
40mm = 40 x 0.1 = 4cm
Volume of the metal = 3 x 0.0003 x 4 = 0.0036cm³
1mL = 1cm³; so volume = 0.0036mL
Mass of the metal = 21.4 x 0.0036 = 0.08g
1g = 1000mg
0.08g = 0.08 x 1000 = 80mg