Answer:
Methyl propyl ether protonates at oxygen, and this makes the oxygen a good leaving group for
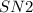 attack by iodide ion which is a good nucleophile.
attack by iodide ion which is a good nucleophile.
We are aware that
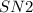 prefers 1 degree carbocation and here both methyl , propyl group is 1 degree.
prefers 1 degree carbocation and here both methyl , propyl group is 1 degree.
So we have two possible carbon atoms to react with iodide which are methyl group and propyl group. They will react with iodide at similar rates, and so there is no great preference of one over the other.
As
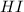 is present in excess the hydrogen of
is present in excess the hydrogen of
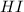 protonates the oxygen which leads to cleavage that can happen on both sides either methyl or propyl side leading to different products.
protonates the oxygen which leads to cleavage that can happen on both sides either methyl or propyl side leading to different products.
The reaction proceeds in the manner
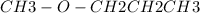 +
+
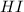 → CH3I + CH3CH2CH2OH
→ CH3I + CH3CH2CH2OH
OR
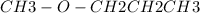 +
+
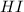 → CH3OH + CH3CH2CH2I
→ CH3OH + CH3CH2CH2I