Answer:
4.3x10⁻⁵ mol/L.
Step-by-step explanation:
Hello there!
In this case, since the dissolution of silver phosphate is not completely achieved due to its low solubility in water, we can represent this as an equilibrium problem by which it separates into silver and phosphate ions:
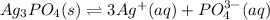
Which are slightly soluble in water; it means that the equilibrium expression is:
![Ksp=[Ag^+]^3[PO_4^(3-)]](https://img.qammunity.org/2021/formulas/chemistry/college/72muqevvjdvn7hi9g04i6yottqiff38nog.png)
Thus, since we know the solubility product and the concentration of these ions in a saturated solution is represented by the reaction extent
 , we can write:
, we can write:
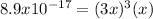
Thus, solving for
 we obtain:
we obtain:
![x=\sqrt[4]{(8.9x10^(-17))/(27) } \\\\x=4.3x10^(-5)M](https://img.qammunity.org/2021/formulas/chemistry/college/w13db0l7nmylv4kkfqjupi65ytzj01odvl.png)
Thus, the molar concentration of silver ions is 4.3x10⁻⁵ mol/L.
Best regards!