Answer:
V₂ = 1518L
Step-by-step explanation:
-Using combined gas law:
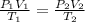
Where P is pressure, V is volume and T is absolute temperature of 1, initial state and 2, final state of the gas.
Initial states:
P₁ = 743 torr
V₁ = 975L
T₁ = 30°C + 273.15K = 303.15K
P₂ = 375 mmHg = torr
V₂ = Our incognite
T₂ = -35°C + 273.15K = 238.15K
Replacing:
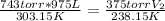
V₂ = 1518L