Answer: The specific heat of the Al is
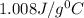 .
.
Step-by-step explanation:
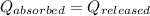
As we know that,
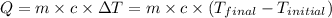
![m_1* c* (T_(final)-T_1)=-[m_2* c* (T_(final)-T_2)]](https://img.qammunity.org/2021/formulas/chemistry/college/z2nwxikp5193qau4vl8mohjbazuhic3vsv.png)
where,
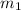 = mass of water = 87.4 g
= mass of water = 87.4 g
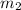 = mass of Al metal = 6.7797 g
= mass of Al metal = 6.7797 g
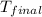 = final temperature =
= final temperature =
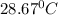
 = temperature of water =
= temperature of water =
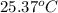
 = temperature of Al metal =
= temperature of Al metal =
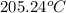
 = specific heat of water =
= specific heat of water =
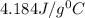
 = specific heat of Al metal = ?
= specific heat of Al metal = ?
Now put all the given values in equation (1), we get
![m_1* c_1* (T_(final)-T_1)=-[m_2* c_2* (T_(final)-T_2)]](https://img.qammunity.org/2021/formulas/chemistry/college/8mq914tycmkoswztk5p5my04anxw73dmzg.png)
![87.4* 4.184* (28.67-25.37)^0C=-[6.7797* c_2* (28.67-205.24)]](https://img.qammunity.org/2021/formulas/chemistry/high-school/m17oizfazwapa7mhjja72patvvpyskgcm8.png)
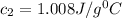
Therefore, the specific heat of the Al is
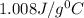 .
.