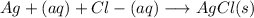Answer:
Follows are the solution to this question
In point 1, the answer is "false".
In point 2, the answer is "True".
Step-by-step explanation:
In point 1:
It's not a response to rain, however, the reaction is also another way.
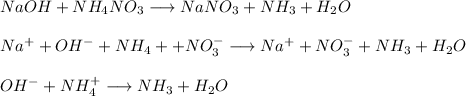
In point 2:
They either use the magnetic resonance or gravimetric analytical method with unknown chloride calculation. They utilize plaster to catalyze chloride through aqueous or salty water. It's also called the response to precipitation.