Number of atoms = number of moles x 6.02 x 10²³
Further explanation
The mole is the number of particles contained in a substance
1 mol = 6.02 x 10²³ particles
mol of Sodium carbonate-Na₂CO₃
MW = 106 g/mol
mass Na
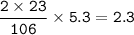
mol Na

number of atoms = 0.1 x 6.02 x 10²³ = 6.02 x 10²²
mass C
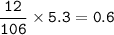
mol C
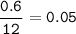
number of atoms = 0.05 x 6.02 x 10²³ = 3.01 x 10²²
mass O

mol O
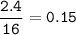
number of atoms = 0.15 x 6.02 x 10²³ = 9.03 x 10²²