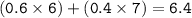The average atomic mass of a sample closer to the mass of Li-6
Further explanation
Isotopes are atoms whose no-atom has the same number of protons while still having a different number of neutrons.
So Isotopes are elements that have the same Atomic Number (Proton)
Atomic mass is the average atomic mass of all its isotopes
In determining the mass of an atom, as a standard is the mass of 1 carbon-12 atom whose mass is 12 amu
Mass atom X = mass isotope 1 . % + mass isotope 2.%
three Li-6, mass number = 6
two Li-7, mass number = 7
total abundance = 5
abundance for Li-6 =
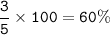
abundance for Li-7 =
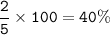
Average atomic mass :