Answer:
320J/g
Step-by-step explanation:
Given parameters:
Mass of the liquid = 40g
Amount of heat added = 12800J
Unknown:
The heat of vaporization = ?
Solution:
The heat of vaporization can be related to heat added by the expression below;
H = mL
H is the heat added
m is the mass
L is the heat of vaporization
Insert the parameters and solve;
12800 = 40 x L
L =
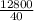 = 320J/g
= 320J/g