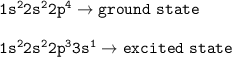2-7-1
Further explanation
Electrons can move the shell up or down by releasing energy or absorbing energy
Excited electrons show higher electron transfer to the shell by absorbing energy
So it can be concluded that there are 2 conditions:
Ground state is the state of electrons filling shell with the lowest energy levels.
Excited state is the state of electrons which occupies a higher energy level
The state of excited electrons can be seen from the presence of electrons which do not fill the skin completely but fill the skin afterward
2-7-1
From its 8 electron configuration, filling 3 shells, 2 electrons in the firs shell, 7 electrons in the second shell and 1 electron in the third shell
the electrons in the third shell should fill the electrons in the second shell first according to Aufbau rule (lower energy shells)