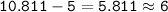Number of neutrons :
Argon=22
Boron=6
Further explanation
There are two components that accompany an element, the mass number and atomic number
Atoms are composed of 3 types of basic particles (subatomic particles): protons, electrons, and neutrons
The Atomic Number (Z) indicates the number of protons and electrons in an atom of an element.
Atomic number = number of protons = number of electrons ⇒ neutral number
Atomic mass is the sum of protons and neutrons
Atomic Number (Z) = Atomic mass (A) - Number of Neutrons
Argon has an atomic number of 18 and a mass number of 39,948
number of neutrons =
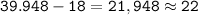
Boron has an atomic number of 5 and a mass number of 10,811
number of neutrons =