Answer:
The thermal efficiency of the power plant cycle is 40.95 percent.
The COP as a refrigerator is 1.442.
Step-by-step explanation:
From Thermodynamics we get that thermal efficiency for power cycles is represented by the following definition:
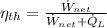 (Eq. 1)
(Eq. 1)
Where:
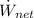 - Net power of the power cycle, measured in kilowatts.
- Net power of the power cycle, measured in kilowatts.
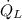 - Heat rate released from condenser, measured in kilowatts.
- Heat rate released from condenser, measured in kilowatts.
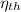 - Thermal efficiency of the power plant cycle, dimensionless.
- Thermal efficiency of the power plant cycle, dimensionless.
The net power cycle is determined by the following expression:
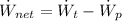 (Eq. 2)
(Eq. 2)
Where:
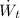 - Power generated by the turbine, measured in kilowatts.
- Power generated by the turbine, measured in kilowatts.
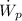 - Power consumed by the pump, measured in kilowatts.
- Power consumed by the pump, measured in kilowatts.
If we know that
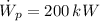 ,
,
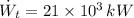 and
and
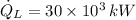 , then the thermal efficiency of the power plant cycle is:
, then the thermal efficiency of the power plant cycle is:
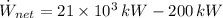
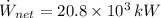
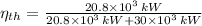
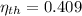 (
(
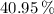 )
)
The thermal efficiency of the power plant cycle is 40.95 percent.
For refrigeration cycles we remember that the Coefficient of Performance (
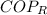 ), dimensionless, is represented by the following model:
), dimensionless, is represented by the following model:
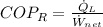 (Eq. 3)
(Eq. 3)
If we know that
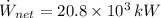 and
and
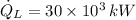 , then the Coefficient of Performance is:
, then the Coefficient of Performance is:
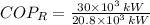
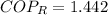
The COP as a refrigerator is 1.442.