Answer: The volume of concentrated acid added will be 4.26 ml
Step-by-step explanation:
The expression used will be dilution law:

where,
 = concentration of stock
= concentration of stock
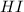 = 6.00 M
= 6.00 M
 = concentration of resulting
= concentration of resulting
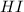 = 0.341 M
= 0.341 M
 = volume of stock
= volume of stock
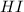 = ?
= ?
 = volume of resulting
= volume of resulting
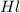 = 75.0 ml
= 75.0 ml
Now put all the given values in the above law, we get the volume of stock
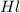 added
added
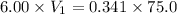
By solving the terms, we get :
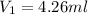
Therefore, the volume of concentrated acid added will be 4.26 ml