Answer:

Step-by-step explanation:
Answer:
Explanation:
A. Volume
The volume was found using water displacement. Subtract the initial volume from the final volume.
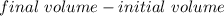
The graduated cylinder had 20 milliliters of water (initial volume).
After the rock was added, the graduated cylinder read 30 milliliters (final volume).
Substitute the values in and subtract.

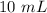
The volume of the rock is 10 milliliters.
B. Density
Density can be found by dividing the mass by the volume.
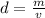
The mass of the rock is 23 grams. We just found the volume of 10 milliliters.
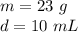
Substitute the values in and divide.
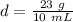
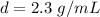
The density of the rock is 2.3 grams per milliliter.