Answer: D
Step-by-step explanation:
I assume you meant
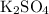 .
.
- The atomic mass of potassium is 39.0983 g/mol.
- The atomic mass of sulfur is 32.065 g/mol.
- The atomic mass of oxygen is 15.9994 g/mol.
So, the formula mass of potassium sulfate is 2(39.0983)+32.065+4(15.9994)=174.2592 g/mol.
So, 5.00 moles have a mass of (5.00)(174.2592), which is about 870 g