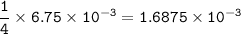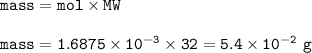mass O₂ required : 0.054 g
Further explanation
The reaction equation is the chemical formula of reagents and product substances
A reaction coefficient is a number in the chemical formula of a substance involved in the reaction equation. The reaction coefficient is useful for equalizing reagents and products
Reaction
4Fe(OH)⁺ (aq) + 4OH⁻ (aq) + O₂ (g)+ 2H₂O (l)⇒ 4Fe(OH)₃ (s)
To precipitate Fe²⁺(in Fe(OH⁺) to Fe³⁺(in Fe(OH)₃)
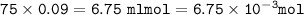
mol ratio from equation = mol O₂ :mol Fe(OH) = 1 : 4