Answer:
8.8kPa
Step-by-step explanation:
Given parameters:
Initial pressure = 8.5kPa
Initial temperature = 295k
New temperature = 305k
Unknown:
New pressure = ?
Solution:
To solve this problem, we have to apply the combined gas law when the volume is constant;
in this instant, when the volume is constant, the pressure of a given mass of gas varies directly with the absolute temperature.
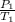 =
=
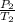
P and T are pressure and temperature
1 and 2 are initial and final states
Insert the parameters and solve;
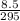 =
=
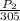
P₂ = 8.8kPa