Answer:
29.38 grams
Step-by-step explanation:
The reaction is:
2N₂(g) + 5O₂(g) → 2N₂O₅ (1)
We need to calculate the number of moles of N₂ and O₂:
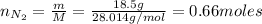
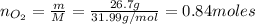
Now, we need to find the limiting reactant. From reaction (1) we have that 2 moles of N₂ react with 5 moles of O₂:
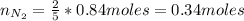
If we have 0.66 moles of N₂ and we need 0.34 moles to react with O₂, then the limiting is O₂.
We can calculate the number of moles of N₂O₅ produced:
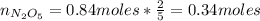
Now, we can calculate the theoretical mass of N₂O₅:
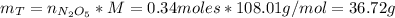
Finally, if the reaction is only 80% efficient then the mass of N₂O₅ produced is:
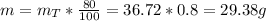
Therefore, are formed 29.38 grams of N₂O₅.
I hope it helps you!