Answer:
The air pressure on a hot day is higher than on a cold day
Step-by-step explanation:
In tire having constant volume, the air pressure on hot day is greater compared to that on a cold day.
From the combined gas law, at constant volume, it can be deduced that the pressure of a given mass of gas varies directly with the absolute temperature.
Mathematically;
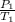 =
=
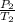
So, it can be inferred that the higher the temperature of the tire, the more the pressure on the air inside and vice versa.