Answer:
a.
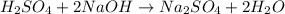
b. 0.092 M
Step-by-step explanation:
Hello!
In this case, since for this titration process we know the used volume of 0.75-M sodium hydroxide needed to neutralize 155 mL of the acid, we first need to write the undergoing chemical reaction between them:
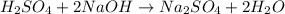
This, since there is 1:2 mole ratio between the acid and the base, at the equivalent point we must respect:
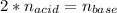
That in terms of concentrations and volumes is:
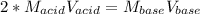
Thus, the concentration of acid was:
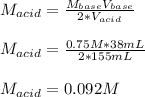
Best regards!