Answer:
200 mL
Step-by-step explanation:
Given that,
Initial volume, V₁ = 300 mL
Initial pressure, P₁ = 0.5 kPa
Final pressure, P₂ = 0.75 kPa
We need to find the final volume of the sample if pressure is increased at constant temperature. It is based on Boyle's law. Its mathematical form is given by :
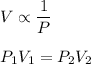
V₂ is the final volume
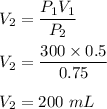
So, the final volume of the sample is 200 mL.