Answer:
The volume will be 12.05 L
Step-by-step explanation:
Boyle's law says that "The volume occupied by a certain gaseous mass at constant temperature is inversely proportional to pressure" and is expressed mathematically as:
P * V = k
Charles's law states that the volume of a gas is directly proportional to the temperature of the gas. That is, Charles's law is a law that says that when the amount of gas and pressure are kept constant, the ratio between the volume and the temperature will always have the same value:
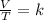
Finally, Gay-Lussac's law states that at constant volume, the pressure of the gas is directly proportional to its temperature. This is expressed mathematically as:
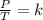
Combined law equation is the combination of three gas laws called Boyle's, Charlie's and Gay-Lusac's law:
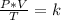
Analyzing an initial state 1 and a final state 2, this law can be expressed as:
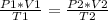
In this case:
- P1= 0.956 atm
- V1= 14.3 L
- T1= 23 °C= 296 °K
- P2= 1.20 atm
- V2= ?
- T2= 40 °C= 313 °K
Replacing:
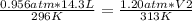
Solving for V2:
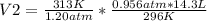
V2= 12.05 L
The volume will be 12.05 L