Answer:
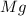 is being oxidized.
is being oxidized.
Step-by-step explanation:
The chemical equation for magnesium metal reacts with hydrochloric acid to produce hydrogen gas and an aqueous solution of magnesium (II) chloride.
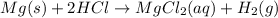
Oxidation reaction : When there is a loss of electrons and an increase in oxidation state number.
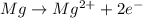
Reduction reaction : when there is gain of electrons and a decrease in oxidation state number.
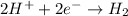
Thus
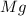 is being oxidized.
is being oxidized.