Answer:
The specific gravity of the mixture is 0.943.
Step-by-step explanation:
The density of the mixture (
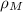 ), measured in kilograms per cubic meters, is defined by the following equation:
), measured in kilograms per cubic meters, is defined by the following equation:
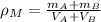 (Eq. 1)
(Eq. 1)
Where:
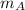 ,
,
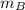 - Masses of liquids A and B, measured in kilograms.
- Masses of liquids A and B, measured in kilograms.
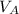 ,
,
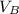 - Volumes of liquids A and B, measured in cubic meters.
- Volumes of liquids A and B, measured in cubic meters.
By applying the definition of density, we expand the density of the mixture as follows:
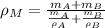
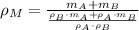
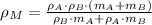 (Eq. 2)
(Eq. 2)
If we know that
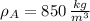 ,
,
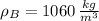 and
and
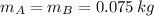 , then the density of the mixture:
, then the density of the mixture:
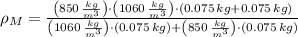
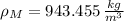
And the specific gravity is the ratio of the density of the mixture to the density of water, that is:
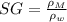 (Eq. 3)
(Eq. 3)
If we get that
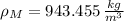 and
and
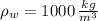 , then the specific gravity of the mixture is:
, then the specific gravity of the mixture is:
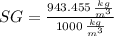
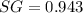
The specific gravity of the mixture is 0.943.