Answer: The atomic weight of the metal would be 85.47.
Step-by-step explanation:
Mass of isotope 1 of metal = 84.9118
% abundance of isotope 1 of metal = 72.15% =
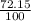
Mass of isotope 2 of metal= 86.9092
% abundance of isotope 2 of metal = 27.85% =
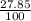
Formula used for average atomic mass of an element :
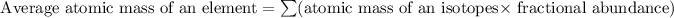
![A=\sum[(84.9118)* (72.15)/(100))+(86.9092)* (27.85)/(100)]]](https://img.qammunity.org/2021/formulas/chemistry/college/lfv8lduntv4xpkc4zvklpb7r38i7ej5y8s.png)
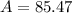
Therefore, the atomic weight of the metal would be 85.47.