Answer:
Magnesium bromide, MgBr2, and water, H2O.
Step-by-step explanation:
Hello!
In this case, since HBr is an acid and Mg(OH)2 is a base, an acid-base reaction is undergone, by which a salt and water are produced as neutralization products:
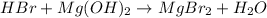
However, it need to be balanced since two bromine atoms are produced, therefore we write:
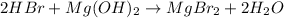
Thus, the products are magnesium bromide, MgBr2, and water, H2O.
Best regards!