Answer:
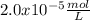
Step-by-step explanation:
Hello!
In this case, since the dissolution of copper (I) chloride is:
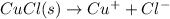
And its equilibrium expression is:
![Ksp=[Cu^+][Cl^-]](https://img.qammunity.org/2021/formulas/chemistry/college/oeqz17hj1zrff7wfzt2ttevs11xgvkdg7i.png)
We can represent the molar solubility via the reaction extent as
 , however, since there is 0.050 M KCl we immediately add such amount to the chloride ion concentration since KCl is readily ionized; therefore we write:
, however, since there is 0.050 M KCl we immediately add such amount to the chloride ion concentration since KCl is readily ionized; therefore we write:
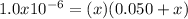
Thus, solving for
 , we obtain:
, we obtain:
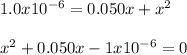
By using the quadratic equation, we obtain:
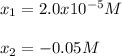
Clearly, the solution is
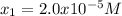 because no negative results are
because no negative results are
allowed. Therefore, the molar solubility is:
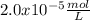
Best regards!