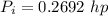Answer:
The required power is
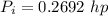
Step-by-step explanation:
From the question we are told that
The temperature of the water entering ice machine is
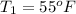
The temperature of the water leaving is
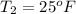
The COP of the ice machine is
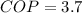
The production rate of an ice
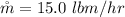
The energy that needs to removed from each lbm of water at 55 F is
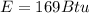
Generally the cooling load of the ice machine is mathematically represented as
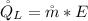
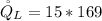
=>
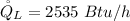
Generally the COP of the ice machine is mathematically represented as
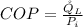
Here
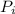 is the net power input needed to successfully run the ice machine
is the net power input needed to successfully run the ice machine
So
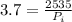
=>
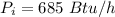
Converting to horsepower
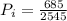
=>