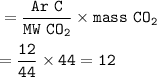C = 12 g
O = 16 g
H = 1 g
Further explanation
Conservation of mass stated that
In a closed system, the masses before and after the reaction are the same
we can calculate the mass of each atom in the compound :
O in O₂ :
mass O₂ = 32
mass O = 32 : 2 = 16 g
H in H₂O
mass H₂O = 18
mass 2.H + mass O = 18
mass 2.H + 16 = 18
mass 2.H=2
mass H = 1 g
C in CH₄
mass CH₄ = 16
mass C + mass 4.H = 16
mass C + 4.1=16
mass C = 12 g
or we can use formula :
Mass of a single C :