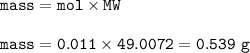mass of NaCN = 0.539 g
Further explanation
A reaction coefficient is a number in the chemical formula of a substance involved in the reaction equation. The reaction coefficient is useful for equalizing reagents and products.
Reaction
2NaCN (s) + H₂SO₄ (aq) ⇒ Na₂S0₄ (aq) + 2HCN (g)
MW HCN = 27,0253 g/mol
MW NaCN = 49,0072 g/mol
Assume there is 1 kg air, and mass of HCN = 300 mg
mol HCN :
mass 300 mg = 0.3 g
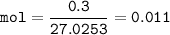
NaCN : HCN = 2 : 2(mole ratio from equation), so :
mol NaCN = mol HCN = 0.011
mass NaCN :