Answer:
0.012mol
Step-by-step explanation:
1.
Given parameters:
Pressure in the balloon = 1.25atm
Volume of the balloon = 2.5L
Unknown:
Number of moles = ?
Solution:
We are going to used the combined gas law to solve this problem.
PV = nRT
P is the pressure
V is the volume
n is the number of moles
R is the gas constant = 0.082atmdm³mol⁻¹K⁻¹
Insert the parameters and solve;
1.25 x 2.5 = n x 0.082 x 285
n = 0.13mol
The number of moles is 0.13mol
2.
Given parameters:
Pressure = 16torr;
to atm is ;
760torr = 1atm
16torr =
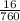 = 0.02atm
= 0.02atm
Volume = 12L
Temperature = 253K
Using the ideal gas law;
PV = nRT
0.02x12 = n x 0.082 x 253
n = 0.012mol