Answer:
There will be left 80.4 of the sample in 500 years
Explanation:
Radioactive Decay Model
Suppose N is the size of a population of radioactive atoms at a given time t, No is the size of an initial population of radioactive atoms at time t = 0, and k is the decay constant, then the equation is as follows:
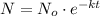
The time required for half of the original population of radioactive atoms to decay is called the half-life Th. The relationship between the half-life and the decay constant is:
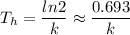
We know the half-life of radium-226 is about 1,590 years. We can find the value of k by solving the above equation:
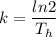
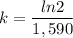
k=0.000436
The initial mass of the sample is 100 gr, thus the model for the mass at time t is:
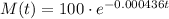
When t=500 years:
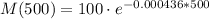
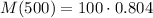
M(500)=80.4 gr
There will be left 80.4 of the sample in 500 years