Answer:
Fe2(SO4)3 has the greater iron content.
Step-by-step explanation:
Hello!
In this case, considering the content of a specific element in a compound, we must take into account the subscript is has in the formula, for instance FeS has one iron atom, Fe2(SO4)3 has two iron atoms and FeCl2 has one iron atom; thus, by assuming one mole of each compound, we can compute the moles of iron there:
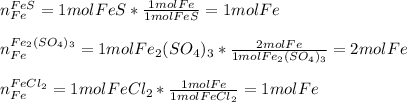
It means that Fe2(SO4)3 would have the greatest iron content.
Best regards!