Answer:
10.01461 kilojoules of thermal energy are absorbed when 98.5 g of water is heated from 24.5 ° C to 48.8 ° C
Step-by-step explanation:
Sensible heat is the amount of heat that a body absorbs or releases without any changes in its physical state (phase change) causing a change in temperature.
The equation that allows to calculate heat exchanges in this case is:
Q = c * m * ΔT
where Q is the heat exchanged by a body of mass m, constituted by a substance of specific heat c and where ΔT is the change in temperature.
In this case:
- c=4.184
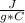
- m= 98.5 g
- ΔT= Tfinal - Tinitial= 48.8°C - 24.5°C= 24.3 °C
Replacing:
Q= 4.184
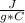 *98.5 g* 24.3 °C
*98.5 g* 24.3 °C
Solving:
Q=10,014.61 J
Since 1 J is equal to 0.001 kJ, then you can apply the following rule of three: if 1 J is equal to 0.001 kJ, then 10,014.61 J is equal to how many kJ?
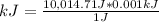
kJ=10.01461 kJ
10.01461 kilojoules of thermal energy are absorbed when 98.5 g of water is heated from 24.5 ° C to 48.8 ° C