Answer:
744.0 mL
Step-by-step explanation:
Formula obtained from Charles Law :
⇒
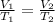
===============================================================
Given :
⇒ V₁ = 780.0 mL
⇒ T₁ = 30 °C = 303 K
⇒ T₂ = 16 °C = 289 K
=============================================================
Solving :
⇒ V₂ = 780 × 289 / 303
⇒ V₂ = 225420 / 303
⇒ V₂ = 744.0 mL (approximately)