Answer:
0.082L or 82mL
Step-by-step explanation:
Given parameters:
Pressure of gas = 780mmHg;
760mmHg = 1atm
780mmHg =
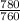 = 1.03atm
= 1.03atm
Volume of gas = 80ml = 0.08L
Standard pressure = 1atm
Unknown:
Volume of gas = ?
Solution:
To solve this problem, we apply Boyle's law which states that "the volume of a fixed mass of gas is inversely proportional to the pressure if the temperature is constant".
Mathematically;
P₁ V₁ = P₂ V₂
P and V are temperature values
1 and 2 are initial and final states;
Insert the parameters and solve;
1.03 x 0.08 = 1 x V₂
V₂ = 0.082L or 82mL