Answer:
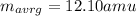
Step-by-step explanation:
Hello.
In this case, for the given natural occurring isotopes, which are those found for that element in nature, in order to compute its average atomic mass we must consider the atomic mass of each isotope and their abundance as shown below:
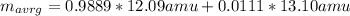
The result is:
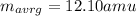
Notice that for carbon, the two stable natural occurring isotopes are C-12 and C-13 in which the predominant one is the C-12, that is why the average atomic mass of carbon is 12.01 amu which is found in the periodic table.
Best regards!