Answer:
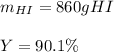
Step-by-step explanation:
Hello.
In this case, for the reaction:
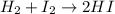
As 6.79 g of hydrogen (molar mass = 2.02 g/mol) react in excess iodine, we can compute the theoretical yield of hydrogen iodide (molar mass = 127.91 g/mol) via their 1:2 mole ratio shown on the chemical reaction:
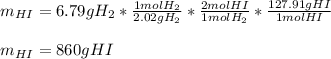
Next, we compute the percent yield by divinding the actual yield (775 g) by the theoretical yield (860 g):
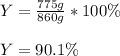
Best regards!